Adverse reaction profile5
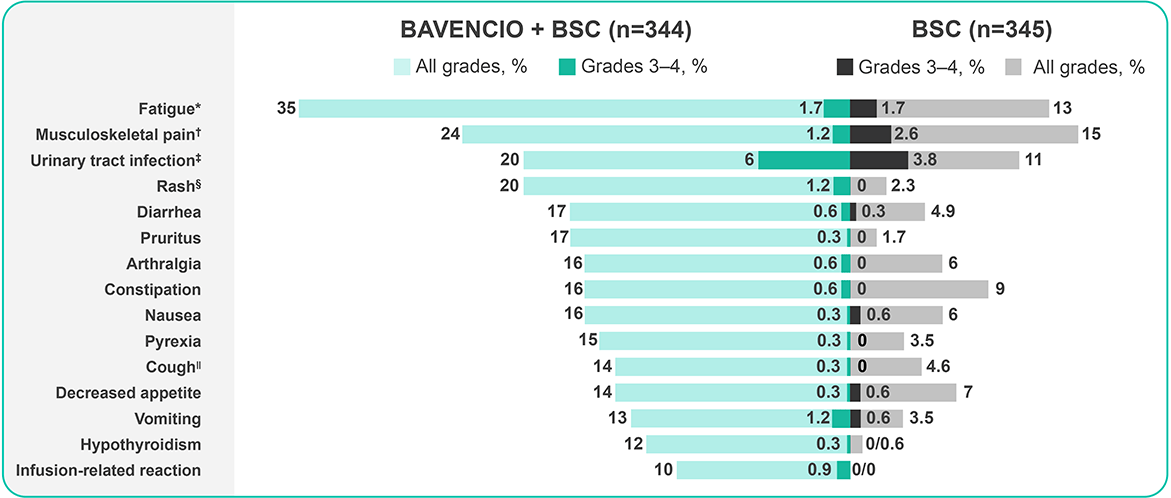
Primary analysis, data cutoff October 21, 20194
Adverse reactions (≥10%) of patients receiving BAVENCIO + BSC

BAVENCIO can cause severe and fatal immune-mediated adverse reactions in any organ system or tissue and at any time after starting treatment with a PD-1/PD-L1 blocking antibody, including after discontinuation of treatment.
Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of PD-1/PD-L1 blocking antibodies.
No dose reduction for BAVENCIO is recommended. For immune-mediated adverse reactions, withhold or permanently discontinue BAVENCIO, depending on severity.
Upon improvement to Grade ≤1, initiate corticosteroid taper and continue to taper over ≥1 month
Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy
Toxicity management guidelines for adverse reactions that do not necessarily require systemic corticosteroids (eg, endocrinopathies and dermatologic reactions) are discussed in subsequent sections
BAVENCIO can cause immune-mediated pneumonitis.
BAVENCIO can cause immune-mediated colitis.
BAVENCIO can cause hepatotoxicity and immune-mediated hepatitis.
BAVENCIO can cause primary or secondary immune-mediated adrenal insufficiency.
BAVENCIO can cause immune-mediated hypophysitis.
BAVENCIO can cause immune-mediated thyroid disorders.
BAVENCIO can cause immune-mediated type I diabetes mellitus, which can present with diabetic ketoacidosis.
BAVENCIO can cause immune-mediated nephritis with renal dysfunction.
BAVENCIO can cause immune-mediated dermatologic adverse reactions, including rash or dermatitis.
BAVENCIO can result in other immune-mediated adverse reactions.
BAVENCIO can cause severe or life-threatening infusion-related reactions.
Fatal and other serious complications of allogeneic hematopoietic stem cell transplantation (HSCT) can occur in patients who receive HSCT before or after being treated with a PD-1/PD-L1 blocking antibody.
BAVENCIO can cause fetal harm when administered to a pregnant woman.
of patients in the BAVENCIO + BSC arm (n=344) permanently discontinued treatment due to an adverse reaction
of patients in the BAVENCIO + BSC (n=344) arm experienced a dose interruption due to an adverse reaction (excluding temporary interruptions of BAVENCIO infusion due to infusion-related reactions)
BSC=best supportive care.


Premedicate patients with an antihistamine and with acetaminophen prior to the first 4 infusions
Administer premedication for subsequent BAVENCIO doses based on clinical judgment and presence and severity of prior infusion reactions
1. Cathomas R, Lorch A, Bruins HM, et al. The 2021 updated European Association of Urology guidelines on metastatic urothelial carcinoma. Eur Urol. 2022;81(1):95-103. doi:10.1016/j.eururo.2021.09.026 2. Galsky M, Balar A, Black P, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of urothelial cancer. J Immunother Cancer. 2021;9:e002552. doi:10.1136/jitc-2021-002552 3. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Bladder Cancer V.3.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed May 25, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 4. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218-1230. 5. Bavencio. Prescribing Information. EMD Serono, Inc.; 2023. 6. Grivas P, Park SH, Voog E, et al. Avelumab first-line maintenance therapy for advanced urothelial carcinoma: comprehensive clinical subgroup analyses from the JAVELIN Bladder 100 Phase 3 Trial. Eur Urol. 2023;84(1):95-108. 7. Powles T, Park SH, Caserta C, et al. Avelumab first-line maintenance for advanced urothelial carcinoma: results from the JAVELIN Bladder 100 Trial after ≥2 years of follow-up. J Clin Oncol. 2023;41(19):3486-3492. 8. Data on file. EMD Serono, Inc., Rockland, MA. 9. US Food and Drug Administration. FDA approves avelumab for urothelial carcinoma maintenance treatment. News release. June 30, 2020. Accessed December 20, 2022. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approvesavelumab-urothelial-carcinoma-maintenance-treatment 10. US Food and Drug Administration. FDA approves nivolumab for adjuvant treatment of urothelial carcinoma. News release. August 19, 2021. Accessed December 20, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-adjuvant-treatment-urothelial-carcinoma 11. Bajorin D, Witjes J, Gschwend J, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384:2102-2014. doi:10.1056/NEJMoa2034442.

Avelumab maintenance is the ONLY NCCN CATEGORY 1 and PREFERRED immunotherapy option for both cisplatin-eligible and -ineligible patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed on first-line platinum-containing chemotherapy.3
Category 1=Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
NCCN=National Comprehensive Cancer Network® (NCCN®)
Preferred intervention=Interventions that are based on superior efficacy, safety, and evidence; and, when appropriate, affordability.
Primary analysis, data cutoff October 21, 20194
| Fatal adverse reaction | A fatal adverse reaction (sepsis) occurred in one (0.3%) patient receiving BAVENCIO + supportive care (BSC) |
|---|---|
| Serious adverse reactions | Serious adverse reactions occurred in 28% of patients receiving BAVENCIO + BSC. Serious adverse reactions in ≥1% of patients included urinary tract infection (including kidney infection, pyelonephritis, and urosepsis) (6.1%), pain (including abdominal, back, bone, flank, extremity, and pelvic pain) (3.2%), acute kidney injury (1.7%), hematuria (1.5%), sepsis (1.2%), and infusion-related reaction (1.2%) |
| Infusion-related reactions | Patients received premedication with an antihistamine and acetaminophen prior to each infusion. Infusion-related reactions occurred in 10% of patients treated with BAVENCIO + BSC (Grade 3: 0.9%) |
| Oral steroid use | Thirty-one (9%) patients treated with BAVENCIO + BSC received an oral prednisone dose equivalent to ≥40 mg daily for an immune-mediated adverse reaction |


