EXPLORATORY SUBGROUP ANALYSIS: mOS from start of 1L platinum-containing chemotherapy8
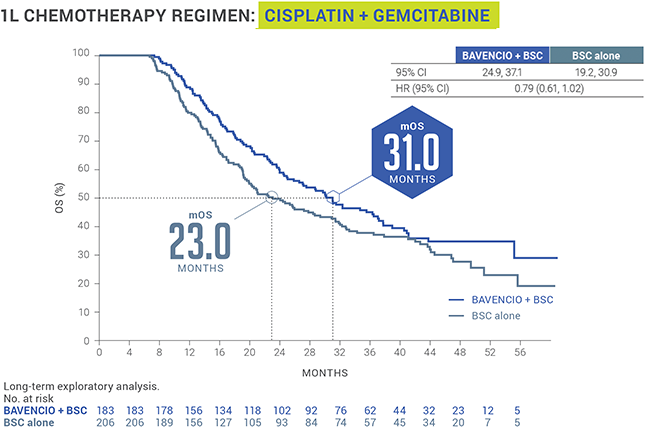
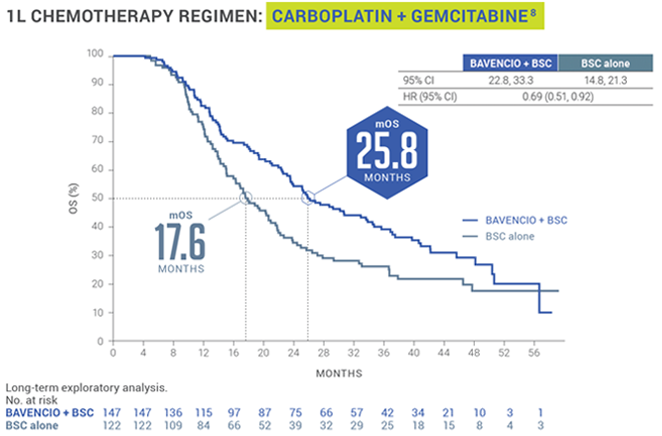
LIMITATIONS: This is an exploratory, post hoc analysis of OS data, inclusive of platinum-containing chemotherapy (4-6 cycles), treatment-free interval (4-10 weeks, per trial protocol), randomized study treatment with BAVENCIO + BSC or BSC alone, and subsequent therapy. This analysis only includes patients who did not progress on first-line platinum-containing chemotherapy and subsequently enrolled in the JAVELIN Bladder 100 trial. Small patient numbers can be a limitation of subgroup analyses. Safety data are not available pre-randomization. No conclusions can be drawn from this OS analysis. 1L=first line; BSC=best supportive care; CI=confidence interval; mOS=median overall survival.






