JAVELIN BLADDER 100 PRIMARY ANALYSIS: BAVENCIO + BSC demonstrated superior OS vs BSC alone5
Median follow-up: 19.6 months (95% CI: 18.0, 20.6) in the BAVENCIO + BSC arm and 19.2 months (95% CI: 17.4, 21.6) in the BSC-alone arm6
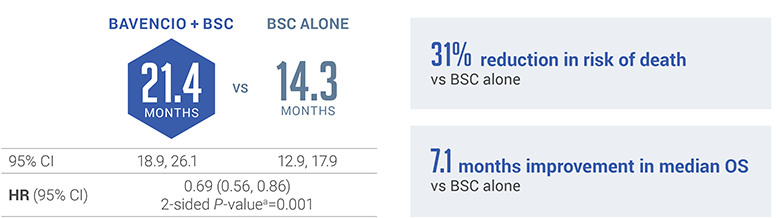
The pre-planned interim analysis was considered the primary analysis since the primary endpoint was met.7,8
LONG-TERM ANALYSIS (3+ years): Consistent OS results were observed5†
Median follow-up: 38.0 months (95% CI: 36.1, 40.5) in the BAVENCIO + BSC arm; 39.6 months (95% CI: 36.2, 41.7) in the BSC-alone arm7,8
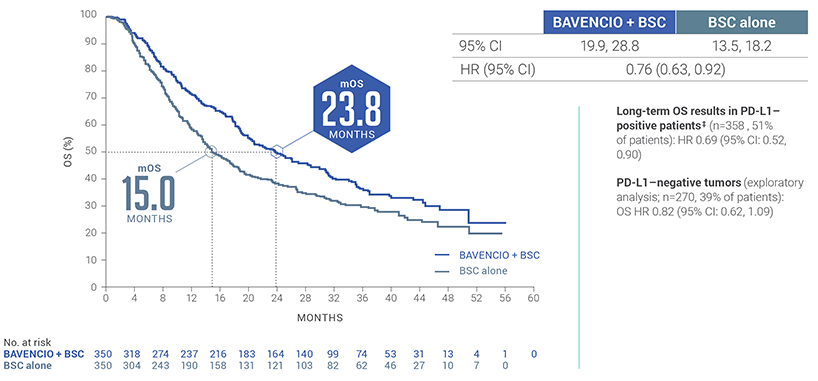
*P-value based on stratified log-rank.4
cells was >1% or ≤1%, respectively. If none of these criteria were met, PD-L1 status was considered negative.4
BSC=best supportive care; CI=confidence interval; CR=complete response; HR=hazard ratio; NE=not estimable; OS=overall survival; PD-L1=programmed death ligand-1.






